Strand7 Software: Case Studies: Skull analysis
Skull analysis: Using Strand7 to Study the Mechanics of Feeding in Mammals |
|
The earliest mammals were tiny, insect-eating creatures. From these unremarkable ancestors, the diversification of mammals is largely a story about exploiting an ever broader array of food resources. Broad associations between the shape of mammals' skulls and their diets are obvious. For example, the architecture of a cow's skull enables it to chew grass efficiently while the skulls of tigers are well equipped to bite and tear flesh. Despite these correlations, we don't understand the mechanistic link between diet and skull shape. The skulls of mammals serve a number of different functions; protecting the brain, housing important sensory organs, and contributing to the first portion of the digestive system. Our lab investigates the hypothesis that skull shape has evolved (at least in part) to withstand the forces that are generated during feeding. We accomplish this by using data on feeding behavior and bite force gathered from wild animals to load finite element models of mammal skulls. By varying the loading conditions, we study how skulls dissipate forces generated by typical and atypical feeding behaviors. We predict that forces are dissipated more efficiently via internal stresses in the skull (i.e., skulls are more resistant to feeding loads) under typical loading condition. Bats are an ideal model organism for this work for three reasons. First, bats exhibit the greatest diversity in skull shape and broadest range of diet among all the orders of mammals. This allows us to compare animals that are closely related but have very different craniofacial structures. Second, the skulls of bats are more likely to be optimized to transmit biting forces than are those of other mammals because the metabolic cost of flight is high. Thus, any tendencies for the skull to be "overbuilt" should have been reduced over evolutionary time. Third, bats are among the most abundant mammals in the world and are relatively easy to work with in the wild. Building a 3-D finite element model of a structure as complicated as a skull poses a significant technical problem. Because the skulls of the bats we study are roughly only 15-20mm long, we turned to micro-ct scanning to capture detailed anatomical structures. Below shows a single slice through the skull of a bat as seen in a micro-ct scan; bone is white. 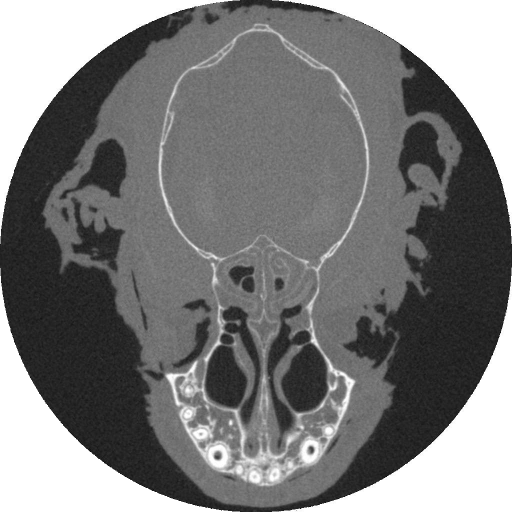
|
|
We built detailed surface models of entire skulls from stacks of serial ct-scans using AMIRA (Mercury Computer Systems). The 3-D surface model of the skull of the Jamaican fruit-eating bat (Artibeus jamaicensis) is shown below. The surface models were saved in STL format and imported into Geomagic (Raindrop, Inc.) where small, particularly complex regions of the skull were edited manually. Once we were satisfied with the geometry, we imported the models to Strand7 as a 3-D surface triangulation (i.e., an *.stl file). Within Strand7 a plate element mesh was automatically constructed from the imported *.stl triangulation. 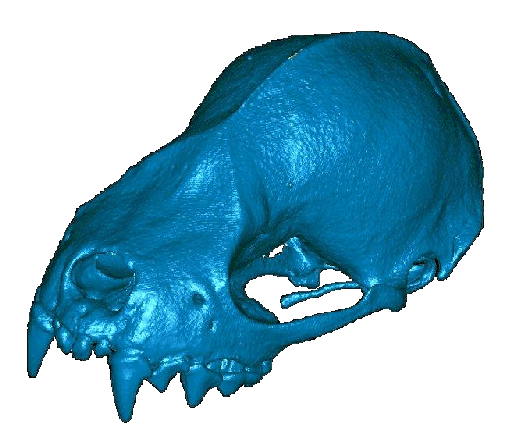
|
|
It is impossible to build such complicated models without errors and Strand7's mesh checking algorithm has proven exceptionally useful. Nevertheless, there are inevitably many plate free edges and t-junctions that must be fixed manually. Once the plate models were free of errors, we used Strand7's automatic tetrahedral mesher to create a volumetric mesh of ten-noded tetrahedrals. After this step, all plate elements were removed, leaving only a volumetric mesh that recreated the geometric structure of the skull in exquisite detail. FE model of the skull containing 251,968 tetrahedral elements is shown below. 
|
|
Applying realistic loads to the models was, of course, crucial to generating meaningful results. We applied load vectors to three nodes representing each of the two primary jaw closing muscles: masseter and temporalis. Constraints were applied at the three places where the lower jaw contacts and transfers forces to the skull during feeding; the center of each jaw joint, and the tip of the tooth where biting occurs. A single node in the center of each joint was constrained against displacement. This effectively created an axis of rotation for the skull due to the application of muscle forces. To prevent this rigid body motion and induce elastic deformation in the skull due to biting forces, nodes on the tips of the teeth involved in biting were constrained against displacement (i.e., displacements in the X, Y, and Z planes were set equal to zero). The point loads representing muscle forces (arrows) and constraints (asterisks) at the biting tooth and jaw joints are illustrated below. 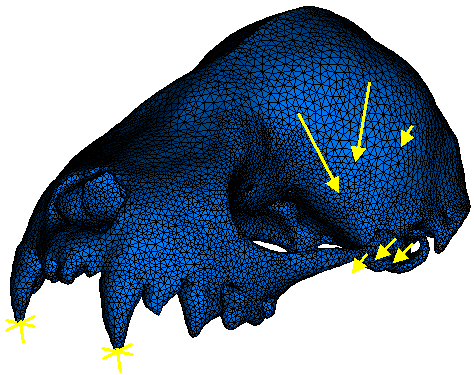
|
|
Each analysis of a biting behavior was completed in two steps. Initially, an arbitrary total amount of muscle force, \(F_T\), was divided between the masseter and temporalis muscles based on muscle mass proportions. All muscles were assumed to act simultaneously and all dynamic or transient effects were neglected. Once the analysis problem was solved, the reaction forces at the constrained tooth required for system static equilibrium were determined. This reaction force, \(F_R^n\), was then compared to experimental in vivo bite force measured for the bat species, \(F_{exp}\). Since the computed reaction force is in direct proportion to the total applied muscle load, the required total amount of muscle force, \((F_T)_{new}\), necessary to yield the experimentally measured bite force is given simply by:
\[(F_T)_{new} = \left({F_{exp} \over F_R^n}\right)F_T\]
In the second step of the analysis, the computed total amount of muscle force \((F_T)_{new}\), was distributed among the masseter and temporalis muscles based on muscle mass portions. The solution of this second analysis problem yielded the deformation of the bat skull, strains, and stresses for a particular feeding behavior that resulted in reaction force(s) at the constrained tooth (teeth) that identically matched voluntary bite force values collected in the field. Essentially, known bite force values were used to calculate the muscle forces required to maintain static equilibrium.
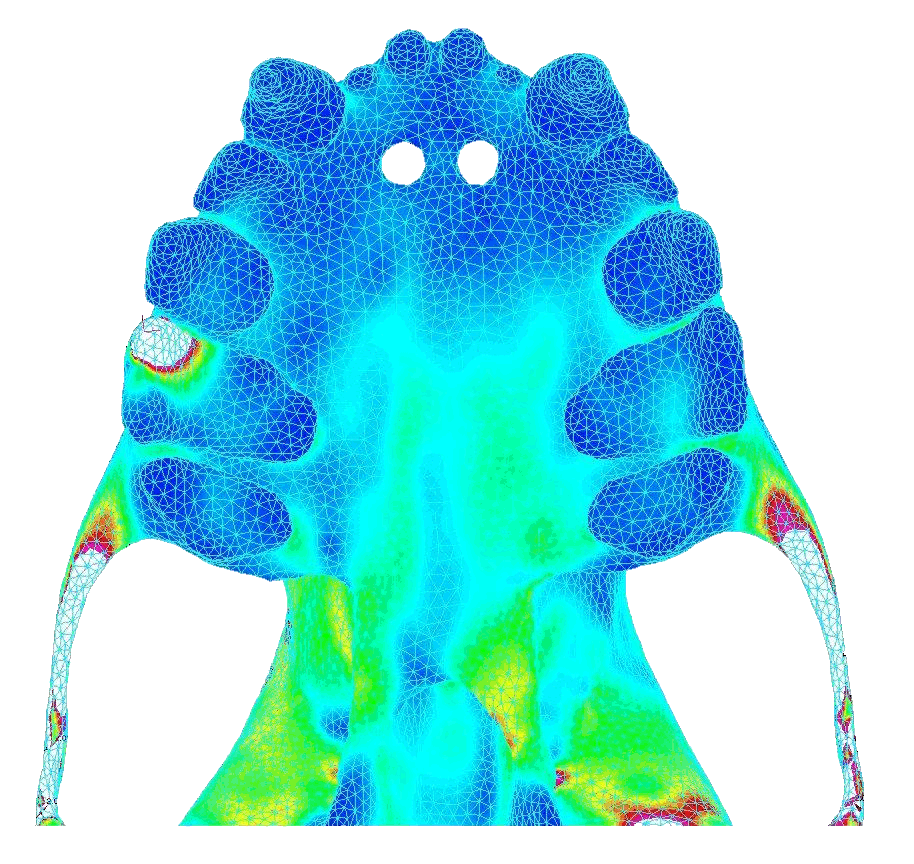
|
|
In contrast, bilateral canine loading resulted in widely distributed stresses that were high and concentrated near the pterygoid plates (toward the back of the palate). Below shows the von Mises stress in the palate under atypical biting behavior (bite force = 18.8 N). 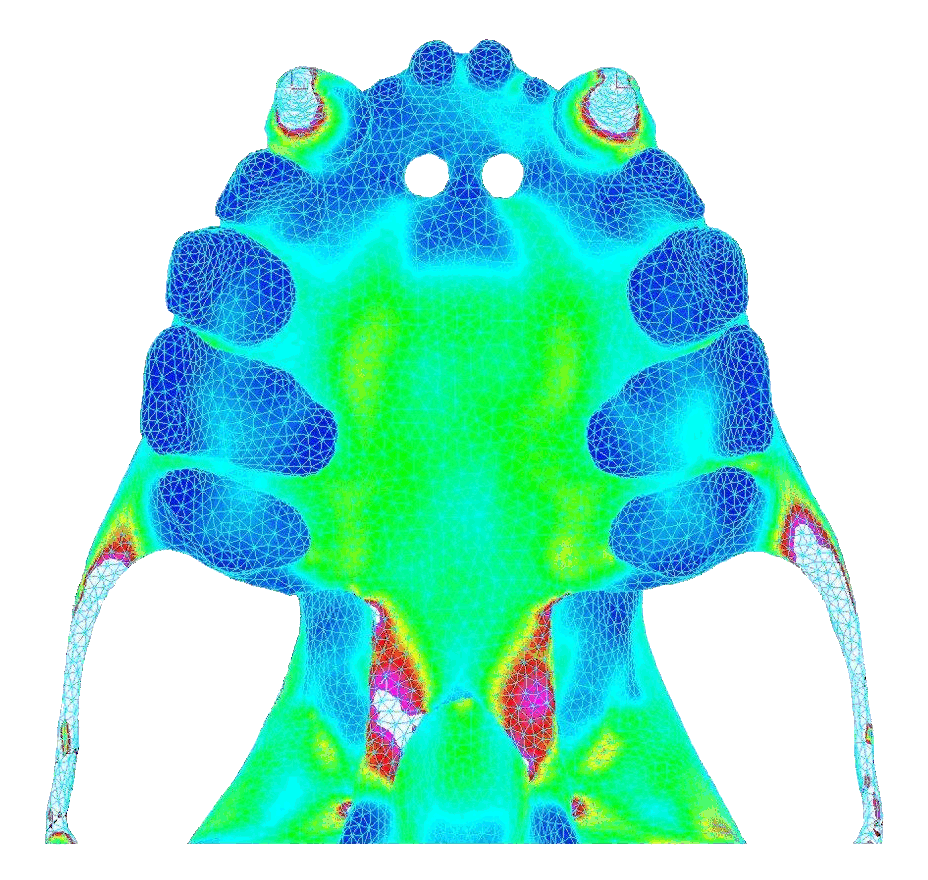
|
|
The ease of extracting quantitative data from Strand7 also allowed us to demonstrate dramatic differences in the volume of the skull experiencing stress under the two loading conditions. After removing the elements affected by the application of point loads, a constant bite force led to a much larger proportion of the skull experiencing high stress under atypical loading compared to the common loading regime as shown below. 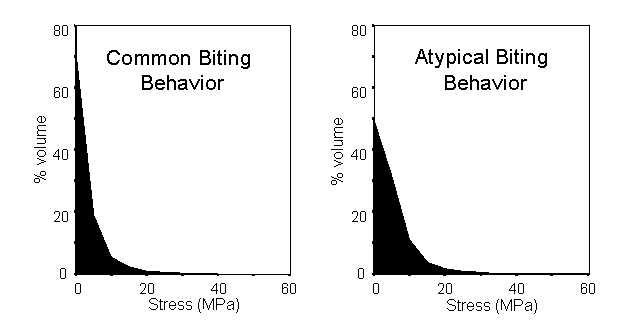
|
|
The bat skull does not constitute a "fully stressed design". Rather, these data support the idea that the skulls of mammals are not optimized solely for feeding but represent a compromise between competing functional demands. Nevertheless, these results demonstrate a clear link between the loading regimes that routinely occur during feeding and the structure of the skull. Based on simple lever mechanics, bite force is expected to increase as bite points move closer to the jaw joints. However, the Jamaican fruit bat exhibits a greater than expected difference in strength between canine and molar biting. We hypothesize that the shape of this bat's skull is a result of natural selection favoring an ever-increasing ability to apply high bite forces. This led to the shortening of the face, focusing biting behaviors on the molar teeth, and strengthening the skull to withstand unilateral molar loads. Article prepared for Strand7 Pty Ltd by Elizabeth Dumont, University of Massachusetts. Research is from the labs of Elizabeth Dumont (Biology) and Ian Grosse (Engineering) at the University of Massachusetts, Amherst USA |

 Menu
Menu